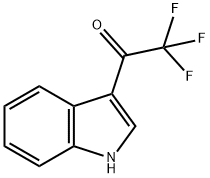
Other grades of this product :
| 3-(TRIFLUOROACETYL)INDOLE Basic information |
| 3-(TRIFLUOROACETYL)INDOLE Chemical Properties |
| Melting point | 211-214 °C(lit.) | | Boiling point | 308.6±37.0 °C(Predicted) | | density | 1.423±0.06 g/cm3(Predicted) | | storage temp. | Sealed in dry,Room Temperature | | form | Solid | | pka | 14.31±0.30(Predicted) | | color | White to Almost white | | CAS DataBase Reference | 14618-45-2(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 3 | | HazardClass | IRRITANT | | HS Code | 2933992000 |
| 3-(TRIFLUOROACETYL)INDOLE Usage And Synthesis |
| Uses | Reactant for enantioselective synthesis of indoles via palladium-catalyzed kinetic asymmetrical alkylation. Reactant for preparation of N-pyridinyl indolecarboxamides via amidation of aminopyridine derivatives. with indolecarboxylic acid 2 Reactant for preparation of mast cell tryptase inhibitors, starting from indoles; using amidation as the key step 3 Reactant for formation of Michael adducts via Baylis-Hillman reaction 4 Reactant for preparation of indolecarboxamides via haloform cleavage of (trifluoroacetyl)indole with lithium dialkylamides. | | Uses | - Reactant for enantioselective synthesis of indoles via palladium-catalyzed kinetic asymmetrical alkylation
- Reactant for preparation of N-pyridinyl indolecarboxamides via amidation of aminopyridine derivatives. with indolecarboxylic acid
- Reactant for preparation of mast cell tryptase inhibitors, starting from indoles; using amidation as the key step
- Reactant for formation of Michael adducts via Baylis-Hillman reaction
- Reactant for preparation of indolecarboxamides via haloform cleavage of (trifluoroacetyl)indole with lithium dialkylamides
| | General Description | 3-(Trifluoroacetyl)indole is a heterocyclic trifluoromethyl ketone. One of the methods reported for its synthesis is by the reaction of indole with trifluoroacetic anhydride. |
| 3-(TRIFLUOROACETYL)INDOLE Preparation Products And Raw materials |
|