Other grades of this product :
| Boc-2-Methoxy-L-Phenylalanine Basic information |
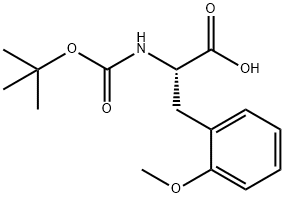
| Product Name: | Boc-2-Methoxy-L-Phenylalanine | | Synonyms: | Boc-2-Methoxy-L-Phenylalanine;Boc-L-Phe(2-OMe)-OH;(Tert-Butoxy)Carbonyl 2-Methoxy-L-Phenylalanine;(2S)-2-{[(tert-butoxy)carbonyl]amino}-3-(2-methoxyphenyl)propanoic acid;L-Phenylalanine, N-[(1,1-dimethylethoxy)carbonyl]-2-methoxy-;Boc-2-methoxy-L-phenylalanine 97%;(S)-2-TERT-BUTHOXYCARBONYLAMINO-3-(2-METHOXY-PHENYL)-PROPIONIC ACID;(S)-2-((tert-Butoxycarbonyl)amino)-3-(2-methoxyphenyl)propanoic acid | | CAS: | 143415-63-8 | | MF: | C15H21NO5 | | MW: | 295.33 | | EINECS: | | Product Categories: | | Mol File: | Mol File |
| Boc-2-Methoxy-L-Phenylalanine Chemical Properties |
| Melting point | 157°C | | storage temp. | 2-8°C | | form | powder |
| Boc-2-Methoxy-L-Phenylalanine Usage And Synthesis |
| Uses | Unnatural amino acid was derived from a C-H Activation methodology developed by Jin-Quan Yu and coworkers. The Pd-mediated C-C bond formation can be made in tandem with 2-Methylpyridine (Aldrich 109835). |
| Boc-2-Methoxy-L-Phenylalanine Preparation Products And Raw materials |
|