Other grades of this product :
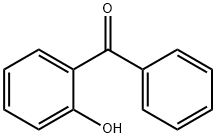
| 2-Hydroxybenzophenone Basic information |
| 2-Hydroxybenzophenone Chemical Properties |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-37/39-36 | | WGK Germany | 3 | | TSCA | Yes | | HazardClass | IRRITANT | | HS Code | 29145000 |
| 2-Hydroxybenzophenone Usage And Synthesis |
| Chemical Properties | yellow crystalline powder | | Uses | UV absorber in plastics. | | Uses | 2-Hydroxybenzophenone was used to quantify the metabolites of unmethylated extracts from the supernatants of Pseudomonas acidovorans cultures grown on 1,1-diphenylethylene. | | Application | 2-Hydroxybenzophenone was used to quantify the metabolites of unmethylated extracts from the supernatants of Pseudomonas acidovorans cultures grown on 1,1-diphenylethylene.2-Hydroxybenzophenone forms copolymer 2-hydroxy, 3-allyl, 4,4′-dimethoxybenzophenone with methyl methacrylate.It forms photostabilizer compounds with 2,2,6,6-tetramethylpiperidine under phase transfer catalysis conditions.It reacts with 3-aminophenyl boronic acid to form macrocyclic boronates. | | Preparation | Preparation by Friedel–Crafts acylation of benzene in the presence of aluminium chloride, ? with 2-hydroxybenzoyl chloride (salicylic acid chloride), (52%), (39%), at temperature <60°; ? with o-anisoyl chloride. Demethylation occurred during the Friedel–Crafts acylation, especially in the presence of ferric chloride at 130–140°; ? with 2-ethoxybenzoyl chloride in a boiling water bath (42%). | | Synthesis Reference(s) | Journal of the American Chemical Society, 76, p. 5469, 1954 DOI: 10.1021/ja01650a062The Journal of Organic Chemistry, 32, p. 3844, 1967 DOI: 10.1021/jo01287a027 | | General Description | - 2-Hydroxybenzophenone forms copolymer 2-hydroxy, 3-allyl, 4,4′-dimethoxybenzophenone with methyl methacrylate.
- It forms photostabilizer compounds with 2,2,6,6-tetramethylpiperidine under phase transfer catalysis conditions.
- It reacts with 3-aminophenyl boronic acid to form macrocyclic boronates.
|
| 2-Hydroxybenzophenone Preparation Products And Raw materials |
|