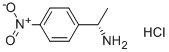Other grades of this product :
| (S)-ALPHA-METHYL-4-NITROBENZYLAMINE HYDROCHLORIDE Basic information |
| (S)-ALPHA-METHYL-4-NITROBENZYLAMINE HYDROCHLORIDE Chemical Properties |
| Melting point | 248-250 °C(lit.) | | refractive index | -7.2 ° (C=1, 0.05mol/L NaOH) | | storage temp. | Inert atmosphere,Room Temperature | | form | powder to crystal | | color | White to Light yellow | | optical activity | [α]25/D 6.5°, c = 1 in 0.05 M NaOH | | Water Solubility | faint turbidity | | CAS DataBase Reference | 132873-57-5(CAS DataBase Reference) |
| (S)-ALPHA-METHYL-4-NITROBENZYLAMINE HYDROCHLORIDE Usage And Synthesis |
| Uses | (S)-α-Methyl-4-nitrobenzylamine hydrochloride can be used as:- A fluorescence-quenching guest in the determination of chiral recognition capabilities of binaphthocrown ether and polythiophene complex.
- A starting material in the synthesis of chiral cyclopalladated complexes with applications in the resolution of racemic compounds.
| | Purification Methods | To ensure dryness, the hydrochloride (ca 175 g) is extracted with EtOH (3x100mL) and evaporated to dryness (any residual H2O increases the solubility in EtOH and lowers the yield). The hydrochloride residue is triturated with absolute EtOH and dried in vacuo. The product is further purified by refluxing with absolute EtOH (200 mL for 83g) for 1hour, and cool to 10o to give 76.6g of hydrochloride m 243-245o(dec). The free base is prepared by dissolving in N NaOH, extracting with CH2Cl2 (3 x 500mL), drying (Na2CO3), filtering, evaporating and distilling it. It has m 27o, b 119-120o/0.5mm (105-107o/0.5mm, 157-159o/9mm, d 1.1764, n 1.5688, [] ±17.7o (neat) [Perry et al. Synthesis 492 1977,ORD: Nerdel & Liebig Justus Liebigs Ann Chem 621 142 1959]. [Beilstein 12 IV 2451.] |
| (S)-ALPHA-METHYL-4-NITROBENZYLAMINE HYDROCHLORIDE Preparation Products And Raw materials |
|