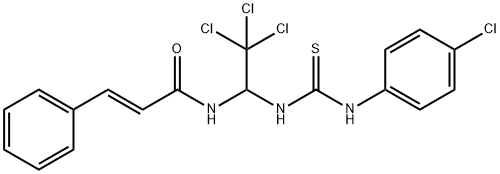
| Uses | Sal003 was used to study the role of eIF2α in Subtilase cytotoxin-induced apoptosis in HeLa cells. |
| Biological Activity | eukaryotic initiation factor 2 α-subunit (eif2α) plays a critical role in the regulation of protein synthesis. moreover, it also plays an important role in synaptic plasticity and long-term memory consolidation. sal003 is a selective inhibitor of eif2α dephosphorylation. |
| Biochem/physiol Actions | Sal003 inhibits the late long-term potentiation (L-LTP) and long-term memory formation in mammalian hippocampal slices. The effect of Sal003 on L-LTP is mediated by the transcription factor, ATF4. |
| in vitro | sal003 specifically prevents dephosphorylation of eif2α by blocking eif2α phosphatases. in sal003-treated cells polysomes dissociated and consistent with this β-actin mrna shifted to lighter fractions, reflecting the inhibition of general translation [1]. |
| in vivo | intra-basolateral amygdala (bla) infusions of sal003 immediately after memory retrieval disrupted the reconsolidation of morphine- or cocaine-induced cpp, leading to a long-lasting suppression of drug-paired stimulus-induced craving. moreover, inhibition of eif2α dephosphorylation in the bla immediately after light/tone stimulus retrieval decreased subsequent cue-induced heroin-seeking behavior in the self-administration procedure. these results demonstrate that eif2α dephosphorylation in the bla mediates the memory reconsolidation of drug-paired stimuli [1]. |
| references | [1] jian m, luo yx, xue yx, han y, shi hs, liu jf, yan w, wu p, meng sq, deng jh, shen hw, shi j, lu l. eif2α dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. j neurosci. 2014;34(30):10010-21. |