Other grades of this product :
| 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI& Basic information |
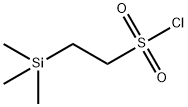
| Product Name: | 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI& | | Synonyms: | 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI&;2-(Trimethylsilyl)ethanesulfonyl chloride;2-Trimethylsilylethylsulfonyl chloride, SES-Cl;2-(triMethylsilyl)ethane-1-sulfonyl chloride;2-(TRISMETHYLSILYL)ETHANESULPHONYLCHLORIDE;SES-Cl;Ethanesulfonylchloride, 2-(triMethylsilyl)-;2-Trimethylsilanyl-ethanesulfonyl chloride | | CAS: | 106018-85-3 | | MF: | C5H13ClO2SSi | | MW: | 200.76 | | EINECS: | | Product Categories: | | Mol File: | 106018-85-3.mol |
| 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI& Chemical Properties |
| Boiling point | 146.8 °C/760 mmHg | | density | 1.059 g/mL at 25 °C | | refractive index | n20/D 1.4444 | | Fp | 21 °C | | storage temp. | 2-8°C | | solubility | Sol most common organic solvents. |
| Hazard Codes | F,Xi | | Risk Statements | 11-36/37/38 | | Safety Statements | 26 | | RIDADR | UN 1993 3/PG 2 | | WGK Germany | 3 | | HS Code | 2931900090 |
| 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI& Usage And Synthesis |
| Physical properties | bp 60 °C/0.1 mmHg; yellow oil. | | Uses | β-Trimethylsilylethanesulfonyl Chloride is used in the protection of primary and secondary amines as their
sulfonamides, which are cleaved by fluoride ion. It participates in the reations of Protection of Amines, SESCl in Synthesis, N-Alkylation, Deprotection Conditions, Formation of 2-Trimethylsilylethanesulfonylimines, the (N-SES-Imino) Phenyliodinane or SESN=IPh Reagent, SES-N3, etc. | | Uses | 2-(Trimethylsilyl)ethanesulfonyl Chloride can be prepared to used to treat cancer and other diseases. | | Preparation | β-Trimethylsilylethanesulfonyl Chloride can be most conveniently synthesized
from commercially available vinyltrimethylsilane (eq 1).
Radical addition of sodium bisulfite to the vinyl group catalyzed
by t-butyl perbenzoate yields the sulfonate salt which can be directly converted to SESCl with phosphorus(V) chloride. The chloride can then be purified by distillation.
The intermediate sulfonate salt is commercially
available. The chloride can also be prepared in 62% yield
from the salt using sulfuryl chloride and triphenylphosphine
(eq 2). A less convenient procedure to synthesize SESCl using β-trimethylsilylethylmagnesium chloride and sulfuryl
chloride has also been developed (eq 3). | | Purification Methods | Check IR; if the bands at ~3200 (OH) cm-1 are strong, then much of the SES-Cl had hydrolysed, and it should be treated with POCl3 (with cooling) and stirred at ~25o for about 1hour, poured into ice cold H2O, extracted with CH2Cl2, washed with NaHCO3, dried (Na2SO4), evaporated , and it distils as a yellow oil in a vacuum. This procedure is used for converting the Na salt to SES-Cl. It reacts with amines to form amides, e.g. SES-NRR’, which on heating with CsF (i.e. F-ions) in DMF at 95o provide the amine (NHRR’), SO2 and CH2=CH2 [Weinreb et al. Tetrahedron Lett 27 2099 1986]. [Ribiere et al. Chem Rev 106 2249 2006.] |
| 2-(TRIMETHYLSILYL)ETHANESULFONYL CHLORI& Preparation Products And Raw materials |
|