Other grades of this product :
| 2,6-Dichlorobenzamide Basic information |
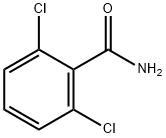
| Product Name: | 2,6-Dichlorobenzamide | | Synonyms: | TIMTEC-BB SBB008056;LABOTEST-BB LT00455181;2,6-DICHLOROBENZAMIDE;ALDRIN PESTANAL (1,2,3,4,10,10-HEXA-CHLO;2,6-DICHLORBENZAMIDE;2,6-BAM;2,6-Dichlorobenzamide,97%;BENZAMIDE,2,6-DICHLORO- | | CAS: | 2008-58-4 | | MF: | C7H5Cl2NO | | MW: | 190.03 | | EINECS: | 217-918-4 | | Product Categories: | Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Alpha sort;D;DAlphabetic;DIA - DIC;Pesticides&Metabolites | | Mol File: | 2008-58-4.mol |
| 2,6-Dichlorobenzamide Chemical Properties |
| 2,6-Dichlorobenzamide Usage And Synthesis |
| Chemical Properties | white to brown-grey crystalline powder | | Uses | 2,6-Dichlorobenzamide is the persistent metabolite of herbicide 2,6-Dichlorobenzonitrile (D431945). | | Definition | ChEBI: A member of the class of benzamides that is benzamide substituted by chloro groups at positions 2 and 6. | | Metabolic pathway | Oral doses of DCB are excreted by rats as DCB, two
monohydroxy-DCBs, 2-chloro-5-hydroxy-6-
(methylthio)benzamide, and 2-chloro-5-hydroxy-6-[S-
(N-acetyl)-cysteinyl]benzamide. Biliary excretion (33%
of the dose), enterohepatic circulation, and intestinal
microfloral metabolism are involved in the formation of
2-chloro-5-hydroxy-6-(methylthio)benzamide. The
major route for the metabolism of DCB is the
conjugation with glutathione in a process involving
phenyl ring hydroxylation at the ortho position to the
S-glutathionyl moiety. Two mechanisms can be
processed for the formation of hydroxylated
metabolites resulting from the epoxidation at the 2-
and 3-positions of the phenyl ring (for the proposed
mechanisms: see the text). |
| 2,6-Dichlorobenzamide Preparation Products And Raw materials |
|