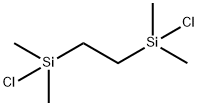
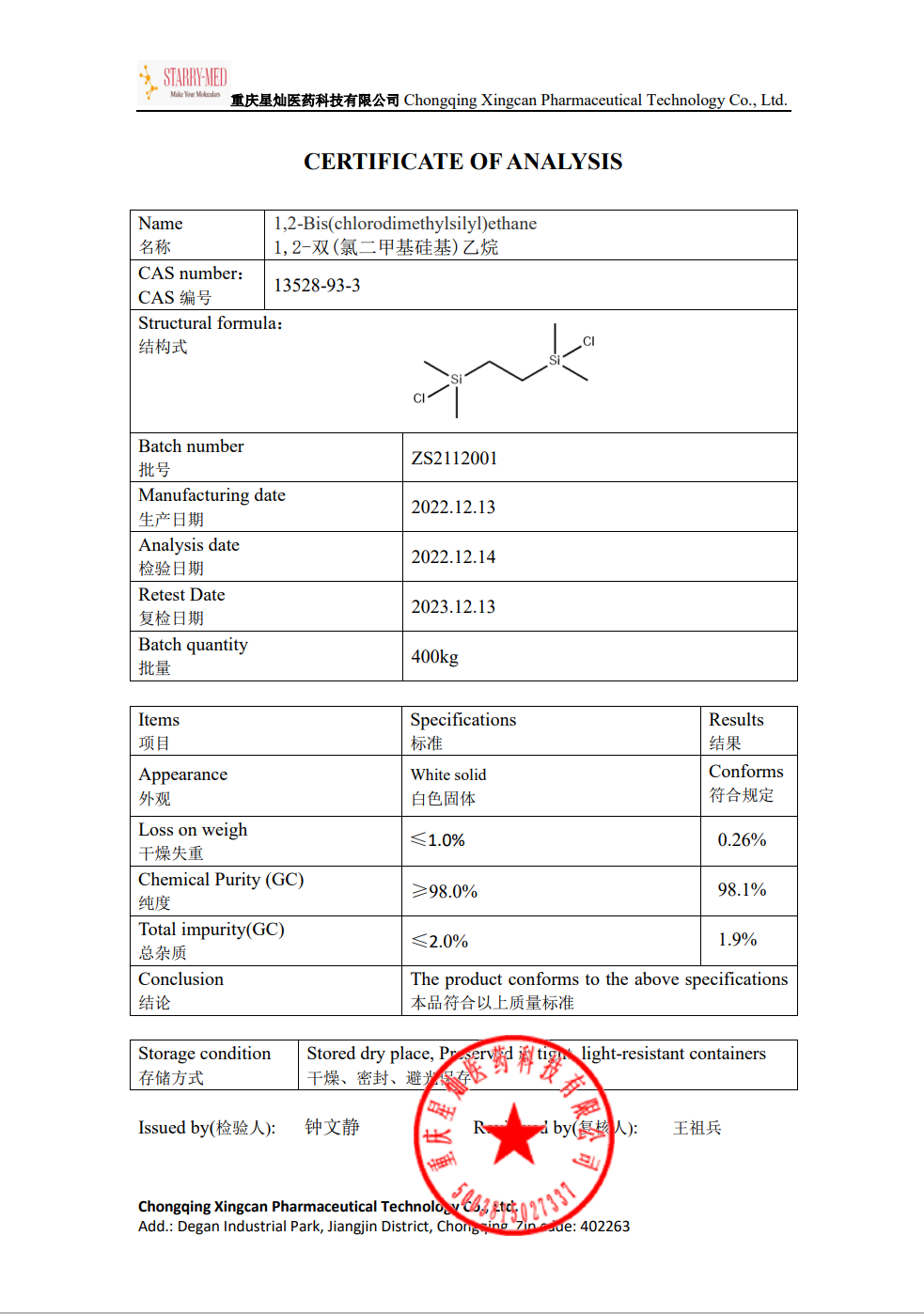
| Melting point | 35-38 °C (lit.) |
| Boiling point | 198 °C/734 mmHg (lit.) |
| density | 0.99 |
| Fp | 155 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Low Melting Solid |
| color | Transparent |
| Water Solubility | Reacts with water. |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1920141 |
| InChIKey | VGQOKOYKFDUPPJ-UHFFFAOYSA-N |
| CAS DataBase Reference | 13528-93-3(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, 1,2-ethanediylbis[chlorodimethyl- (13528-93-3) |
1,2-Bis(chlorodimethylsilyl)ethane can be used as a reagent for the protection of primary amines as their tetramethyldisilylazacyclopentane or Stabase adducts; used in combination with zinc to form organozinc carbenoids; electrophilic silylating reagent.
Protection of Primary Amines.
Few protecting groups exist for the N,N-diprotection of primary amines. The tetramethyldisilylazacyclopentane or Stabase adduct (3) is particularly attractive for this purpose due to its ease of preparation, base stability and facile removal. This cyclic disilane is easily prepared (eq 1) from the commercially available title reagent (1).For primary amines with pKa values between 10 and 11, typical reaction conditions involve the treatment of a dichloromethane solution of the amine with (1) in the presence of two equivalents of triethylamine at room temperature. Subsequent workup with aqueous dihydrogen phosphate provides (3) in excellent yields.1 Stabase adducts are quite stable to strongly basic conditions such as n-butyllithium and s-butyllithium (at ?25°C), lithium diisopropylamide, and Grignard reagents making it a superb candidate for use in the alkylation of substrates bearing a primary amine. For example, protected amino acid derivatives are easily alkylated (eq 2).After formation of the lithium enolate of the protected ethyl glycinate (4) under standard conditions, exposure to various alkyl halides or aldehydes yields alkylation products such as (5).

Please leave a message for us or use the following ways to contact us, we will reply to you as soon as possible, and provide you with the most sincere service, thank you.