Other grades of this product :
| 3,5-Dinitrobenzonitrile Basic information |
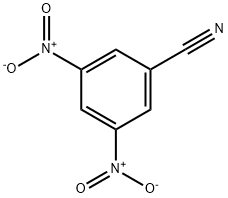
| Product Name: | 3,5-Dinitrobenzonitrile | | Synonyms: | 3,5-dinitro-benzonitril;3,5-Dinitrobenzonitrile 98+%;3,5-DINITROBENZONITRILE;3,5-BISNITROBENZONITRILE;5-CYANO-1,3-DINITROBENZENE;3,5-DINITROBENZONITIRLE;3,5-Dinitrobenzonitrile,97%;3,5-Dinitrobenzonitrile> | | CAS: | 4110-35-4 | | MF: | C7H3N3O4 | | MW: | 193.12 | | EINECS: | 223-889-9 | | Product Categories: | Nitrogen Compounds;Organic Building Blocks;Aromatic Nitriles;C6 to C7;Cyanides/Nitriles;Building Blocks;Nitrogen Compounds;C6 to C7;Chemical Synthesis | | Mol File: | 4110-35-4.mol |
| 3,5-Dinitrobenzonitrile Chemical Properties |
| Melting point | 126-130 °C(lit.) | | Boiling point | 329.31°C (rough estimate) | | density | 1.6504 (rough estimate) | | refractive index | 1.5500 (estimate) | | storage temp. | Sealed in dry,Room Temperature | | Water Solubility | Insoluble in water | | form | powder to crystal | | color | White to Yellow to Green | | CAS DataBase Reference | 4110-35-4(CAS DataBase Reference) | | NIST Chemistry Reference | Benzonitrile, 3,5-dinitro-(4110-35-4) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-36/37/38-36/38 | | Safety Statements | 26-36 | | RIDADR | 3276 | | WGK Germany | 3 | | RTECS | DI4365000 | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 29269090 |
| 3,5-Dinitrobenzonitrile Usage And Synthesis |
| Chemical Properties | yellow crystals or crystalline powder | | Preparation | In a well-ventilated hood, diphosgene (2 mL) was added dropwise to a cold (0–5 C°), stirred solution of 3,5-dinitrobenzamide (2.1 g, 10 mmol) in trimethyl phosphate (6.3 mL). The reaction mixture was then slowly heated to 60 C° for 5 min to ensure completion of the reaction and also to distil off any generated phosgene. After cooling in an ice/water bath, the reaction mixture was vigorously stirred and iced water (10 mL) was added to destroy any traces of phosgene or chloroformate. The precipitated solid product was collected by filtration, washed with water to eliminate traces of HCl and trimethyl phosphate, and air-dried; yield: 1.88 g (96%), mp 127–129 C°. Recrystallization from diisopropyl ether gave an analytically pure product, mp 130–131 C°. The same authors also reported that alkyl, benzylic, aryl, and heteroaryl aldoximes bearing various functionalities could be readily converted to the corresponding nitriles using diphosgene in good yields of 82–96%. | | Synthesis Reference(s) | Tetrahedron Letters, 27, p. 2203, 1986 DOI: 10.1016/S0040-4039(00)84487-1 |
| 3,5-Dinitrobenzonitrile Preparation Products And Raw materials |
|