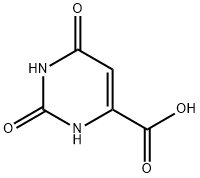
Other grades of this product :
| Melting point | ≥300 °C |
| Boiling point | 280.29°C (rough estimate) |
| density | 1.6814 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| pka | pK1:1.8(+1);pK2:9.55(0) (25°C) |
| form | Crystalline Powder |
| color | White |
| Water Solubility | Slightly soluble |
| Merck | 13,6942 |
| BRN | 383901 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | PXQPEWDEAKTCGB-UHFFFAOYSA-N |
| CAS DataBase Reference | 65-86-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Orotic acid(65-86-1) |
| EPA Substance Registry System | Orotic acid (65-86-1) |
Orotic acid (also known as pyrimidinecarboxylic acid) is a heterocyclic acid; it is also known as. Historically it was believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin. It is well known as a precursor in biosynthesis of pyrimidines; in mammals it is released from the mitochondrial dihydroorotate dehydrogenase (DHODH) for conversion to UMP by the cytoplasmic UMP synthase enzyme[1]. OA is also a normal part of the diet, being found in milk and dairy products[5], and it is converted to uridine for use in the pyrimidine salvage pathway predominantly in liver, kidney and erythrocytes. Before its essential role as an intermediate of pyrimidine biosynthesis was established in the 1950s[3], the compound OA was discovered in whey (Greek oros) by Biscaro and Belloni (1905)[4]. Since then it has received attention from several different aspects. For example, it is proposed that the dietary orotate is beneficial for animals and humans obviously arose from its considerable natural amounts in milk and dairy products[5]. In addition, it also draw great attention from its medical potentials such as improvement of learning behavior of adult rats, neuro-protective effect for gerbils and cats under transient cerebral ischemia, and optimization of functions in normal and ischemic rat hearts[6-9].