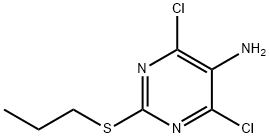
| Synthesis | The synthesis of 4,6-dichloro-2-propylthiopyrimidine-5-amine is as follows:tert-Butyl methyl ether (370 g) was placed under nitrogen in a 1 L stainless steel autoclave equipped with a temperature-controlled jacket, an Ekato InterMIG stirrer, an internal temperature sensor and a dip pipe, and 4,6-dichloro-5-nitro-2-propylsulfanyl-pyrimidine (94.5 g, 0.35 mol) was added and dissolved at a stirring rate of 200 min-1. The catalyst suspension was prepared and transferred into the autoclave as described in the preceding example. The autoclave was sealed and the stirring rate was increased to 600 min-1 while the autoclave was purged four times with nitrogen. Subsequently, hydrogen gas feed via the dip pipe at a constant flow rate (pmax=10 bar) as well as a heating-up ramp (45 K/h) from 20° C. to 65° C. were started in parallel, while stirring at 600 min-1. The progress of the exothermic reaction was followed by recording the hydrogen uptake as well as the internal and jacket temperature curve. Upon completion of the hydrogen uptake (ca. 1.1 mol or 3 molar equivalents) after about 4 h, stirring of the reaction mixture was continued for an additional 3 hours at 65° C. After unloading the autoclave (the reactor was cooled down to 20° C., the hydrogen pressure was released and the reactor purged four times with nitrogen), the catalyst was filtered off. The autoclave as well as the filter cake (catalyst) were washed with tent-butyl methyl ether (185 g). The organic phases were combined and the water layer separated. An IPC-sample was taken to analyze the product mixture. The conversion was found to be quantitative with no nitroso or hydroxylamine intermediate being detectable. |