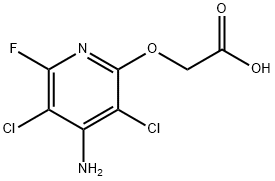
| Chemical Properties | White crystalline solid. |
| Uses | Fluroxypyr is a systemic and selective herbicide. Fluroxypyr is used for the control of broad-leaved weeds in small grain cereals, maize, pastures, range land and turf. Fluroxypyr is a synthetic auxin
. |
| Uses | Fluroxypyr is a systemic and selective herbicide. Fluroxypyr is used for the control of broad-leaved weeds in small grain cereals, maize, pastures, range land and turf. Fluroxypyr is a synthetic auxin. |
| Definition | ChEBI: An aminopyridine that is pyridin-4-amine substituted by chloro groups at positions 3 and 5, a fluoro group at position 6 and a carboxymethoxy group at position 2. |
| Trade name | methyl ester: AGROSTAR; BOFIX FFC®;
CABADEX®; CASCADE®; DOWCO® 433 MHE;
FOREFRONT®; GALAXY GL184®; PARADIGM®;
PASTUREGARD®; STARANE®; TOMAHAWK®;
VISTA®; WIDEMATCH®, (fluroxypyr + clopyralid);
XRM-5084® |
| Potential Exposure | Those who manufacture, distribute or
use this pyridinecarboxylic acid/pyridine herbicide. |
| Environmental Fate | The DT50 in soil is relatively short,
ranging from 5 to 9 days in experiments conducted in
the laboratory. This rapid degradation is due to microbes.Because of its rapid soil degradation, little fluroxypyr is
available for other dissipation processes, e.g., leaching. |
| Metabolism | Fluroxypyr is stable under acidic conditions
and moderately stable in alkaline conditions; e.g., the
DT50 in water at pH 9 is 185 days. Degradation occurs at
temperatures above its melting point. Fluroxypyr is stable
in light.
Plant. The metabolism of fluroxypyr has not been fully
described. Conjugates of fluroxypyr have been observed in
tolerant plants.
Soil. Fluroxypyr is rapidly metabolized by soil
microbes via the removal of the carboxyl group or
acetic acid group, yielding 4-amino-3,5-dichloro-6-fluoro-
2-methoxypyridine, and 4-amino-3,5-dichloro-6-fluoro-2-
pyridinol, respectively. |
| Shipping | UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous haz ardous material, Technical Name Required. |
| Toxicity evaluation | Mammalian Toxicity. Fluroxypyr appears to be rapidly
excreted, unmodified, in the urine of animals. The acute
oral LD50 for rat is 2405 mg/kg.
Weed Resistance/Modified Crop Tolerance. Fuerst et al.
(48) reported fluroxypyr resistance in yellow starthistle
(Centaurea solstitialis). No crops with modified tolerance
toward fluroxypyr are currently in production. |
| Incompatibilities | May not be compatible with nitrates.
Moisture may cause hydrolysis or other forms of decompo sition; forming a strong acid. Temperatures above 250℃ can cause decomposition. Methyl ester: Esters react with
acids to liberate heat along with alcohols and acids. Strong
oxidizing acids may cause a vigorous reaction that is suffi ciently exothermic to ignite the reaction products. Heat is
also generated by the interaction of esters with caustic solu tions. Flammable hydrogen is generated by mixing esters
with alkali metals and hydrides. |
| Waste Disposal | Containers must be disposed
of properly by following package label directions or by
contacting your local or federal environmental control
agency, or by contacting your regional EPA office.
Dissolve or mix the material with a combustible solvent
and burn in a chemical incinerator equipped with an after burner and scrubber. In accordance with 40CFR165, follow
recommendations for the disposal of pesticides and pesti cide containers. |