Other grades of this product :
| Ranitidine Basic information |
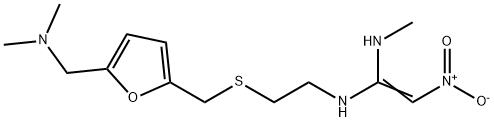
| Product Name: | Ranitidine | | Synonyms: | (E)-N1'-[2-[[5-[(dimethylamino)methyl]-2-furanyl]methylthio]ethyl]-N1-methyl-2-nitroethene-1,1-diamine;1,1-Ethenediamine, N-[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-;1,1-ethenediamine,n-(2-(((5-((dimethylamino)methyl)-2-furanyl)methyl)thio)eth;RANITIDINE;RANITIDINE BASE;TIMTEC-BB SBB006527;N’-Methyl-N-[2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethyl]-2-nitro-1-ethenediarnine;Sostril | | CAS: | 66357-35-5 | | MF: | C13H22N4O3S | | MW: | 314.4 | | EINECS: | 266-332-5 | | Product Categories: | API;Active Pharmaceutical Ingredients;66357-35-5 | | Mol File: | 66357-35-5.mol |
| Ranitidine Chemical Properties |
| Ranitidine Usage And Synthesis |
| Description | Ranitidine, a H2-receptor agonist, caused contact
dermatitis within the pharmaceutical industry. | | Uses | Antagonist (to histamine H2receptors). | | Uses | It simultaneously reduces pepsin activity and is used for treating stomach
and duodenum ulcers as well as other conditions accompanied by elevated acidity of the gastrointestinal
tract. Synonyms of this drug are zantac, azantac, raniplex, ranidil, and others. | | Uses | Ranitidine (cas# 66357-35-5) was used as a standard for testing the therapeutic effect of brown propolis extract against aspirin and ethanol- induced gastric ulcers. | | Indications | Ranitidine (Zantac) is another H2 receptor antagonist that does not have the
same antiandrogen side effects as cimetidine. Note that both cimetidine and
ranitidine inhibit the cytochrome P-450 microsomal enzyme system. | | Brand name | Zantac (GlaxoSmithKline). | | General Description | Ranitidine, N-[2-[[[5-(dimethylamino)methyl]-2-furanyl]methyl]thiol] ethyl]-N'-methyl-2-nitro-l,1-ethenediamine (Zantac), is a white solid, which inits hydrochloride salt form is highly soluble in water. It is anaminoalkyl furan derivative with pKa values of 2.7 (sidechain) and 8.2 (dimethylamino). Ranitidine is more potentthan cimetidine, but less potent than famotidine. Likeother H2-antagonists, it does not appear to bind to otherreceptors.Bioavailability of an oral dose of ranitidine is about 50%and is not significantly affected by the presence of food.Some antacids may reduce ranitidine absorption and shouldnot be taken within 1 hour of administration of this drug. Theplasma half-life of the drug is 2 to 3 hours, and it is excretedalong with its metabolites in the urine. Three metabolites, ranitidineN-oxide, ranitidine S-oxide, and desmethyl ranitidine,have been identified. Ranitidine is only a weak inhibitor ofthe hepatic cytochrome isozymes, and recommended doses ofthe drug do not appear to inhibit the metabolism of otherdrugs. However, there have been isolated reports of drug interactions(warfarin, triazolam) that suggest that ranitidinemay affect the bioavailability of certain drugs by someunidentified mechanism, perhaps by pH-dependent effect onabsorption or a change in volume of distribution.In addition to being available in various dosage forms asthe hydrochloride salt, ranitidine is also available as a bismuthcitrate salt for use with the macrolide antibiotic clarithromycinin treating patients with an active duodenalulcer associated with H. pylori infection. Eradication of H.pylori reduces the risk of duodenal ulcer recurrence. | | Biological Activity | Potent, selective and competitive histamine H 2 receptor antagonist (pA 2 = 6.95-7.2). In vivo, inhibits gastric acid secretion induced by histamine, pentagastrin, bethanecol and food. Also inhibits aspirin-induced gastric lesions. | | Contact allergens | Ranitidine, an H2-receptor antagonist, can cause contact
dermatitis within the pharmaceutical industry and
in health care workers, or may induce systemic drug
reactions in patients. | | Clinical Use | H2
antagonist:
Conditions associated with hyperacidity | | Synthesis | Ranitidine, N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-
N′-methyl-2-nitro-1,1-ethendiamine (16.2.8), is synthesized from furfuryl alcohol, which
undergoes aminomethylation reaction using dimethylamine and paraform, which form 5-
(dimethylaminomethyl)furfuryl alcohol (16.2.6). Further reaction with 2-mercaptoethylamine
hydrochloride gives a product of substitution of the hydroxyl group in (16.2.6),
5-dimethylaminomethyl-2-(2′-aminoethyl)thiomethylfurane (16.2.7). Reacting this with Nmethyl-
1-methylthio-2-nitroethenaamine gives ranitidine (16.2.8). | | Drug interactions | Potentially hazardous interactions with other drugs
Alpha-blockers: effects of tolazoline antagonised.
Antifungals: absorption of itraconazole and
ketoconazole reduced; concentration of posaconazole
possibly reduced - avoid.
Antivirals: concentration of atazanavir reduced;
concentration of raltegravir possibly increased - avoid;
avoid for 12 hours before and 4 hours after rilpivirine.
Ciclosporin: may increase or not change ciclosporin
levels; nephrotoxicity, additive hepatotoxicity and
thrombocytopenia reported.
Cytotoxics: reduced gefitinib concentration; reduces
concentration of erlotinib and possibly pazopanib,
give at least 2 hours before or 10 hours after
ranitidine; absorption of dasatinib reduced - avoid;
possibly reduced absorption of lapatinib.
Ulipristal: contraceptive effect possibly reduced -
avoid with high dose ulipristal. | | Metabolism | Ranitidine is not extensively metabolised. A small
proportion of ranitidine is metabolised in the liver to
the N-oxide, the S-oxide, and desmethylranitidine; the
N-oxide is the major metabolite but accounts for only
about 4-6% of a dose.
The fraction of the dose recovered as metabolites is
similar after both oral and IV dosing; and includes 6%
of the dose in urine as the N-oxide, 2% as the S-oxide,
2% as desmethylranitidine and 1-2% as the furoic acid
analogue. There is also some excretion in the faeces. | | Dosage forms | 150 mg b.i.d. |
| Ranitidine Preparation Products And Raw materials |
|